4 Innovations in Chronic Whiplash Therapy
February 16, 2024
9 min. read

Whiplash injuries, commonly resulting from motor vehicle collisions (MVC), present a complex challenge for physical therapists aiming to optimize patient recovery—especially when neck and bodily pain becomes chronic. Because the critical period for intervention typically falls within the first 12 weeks post-injury, it's especially important to perform early and thorough assessments on a patient-by-patient basis.
In this article, we'll take a look at some well-established techniques for doing so as well as some key innovations in the assessment and treatment of patients with whiplash injury (whether from an MVC or another event such as a sports match).
Common High-Risk Indicators for Chronic Whiplash
The recovery trajectory for patients with whiplash has a wide range, with some improving rapidly and others experiencing ongoing chronic neck and bodily pain. Recognizing this diversity is key in tailoring interventions to individual patient needs.
To help forecast potential chronic pain, it's important to understand the high-risk indicators. Factors such as high initial neck pain intensity and neck-related disability, elevated post-traumatic stress symptoms, and cognitive concerns about chronic pain have been consistently linked to an increased risk of a protracted recovery.1
Prognostic and widely available tools, as detailed in the Ritchie algorithm or more generic tools like the Traumatic Injuries Distress Scale, offer valuable insights into predicting recovery pathways. Physical therapists can leverage these clinical tools to inform treatment strategies, helping to differentiate between and monitor patients likely to recover uneventfully and those at risk of prolonged disability.
Innovations in Chronic Whiplash Therapy
As the field of chronic whiplash treatment continues to evolve, it's essential for clinicians to stay informed about new research and innovations that have the potential to translate into clinical practice. With that in mind, let's delve into three emerging strategies that we believe show promise for helping clinicians identify why a patient might be experiencing chronic whiplash.
1. Leveraging Artificial Intelligence
In our ongoing spinal pain research, we've found profound changes in the muscle composition of patients who transition from acute to chronic whiplash, in tandem with the previously stated risk factors. These changes, which have been shown to occur in three different countries with different insurance schemes,2,3,4 occur primarily in the deeper muscles close to the spine. These are not as common in healthy controls, in patients with idiopathic non-traumatic neck pain,5,6 or in those who have recovered from chronic neck pain.
However, because identifying these changes on a clinically warranted MRI or a CT scan takes hours, we know that it is unlikely this will ever translate to clinical practice. While we're not endorsing that all patients need imaging, some do; and if they do, novel measures can now be leveraged to reveal changes never before realized.
For example, our multidisciplinary team has developed multi-muscle deep learning segmentation algorithms to automate the quantification. What used to take four to eight hours now takes minutes, which improves the potential to implement these methods into busy clinical environments where time is of the essence. These algorithms allow for the accurate and rapid quantitative assessment of the composition of the architecturally complex muscles that traverse the cervical spine,7 and we've also created one for the lumbar spine.8
2. Assessing Possible Central Nervous System Involvement
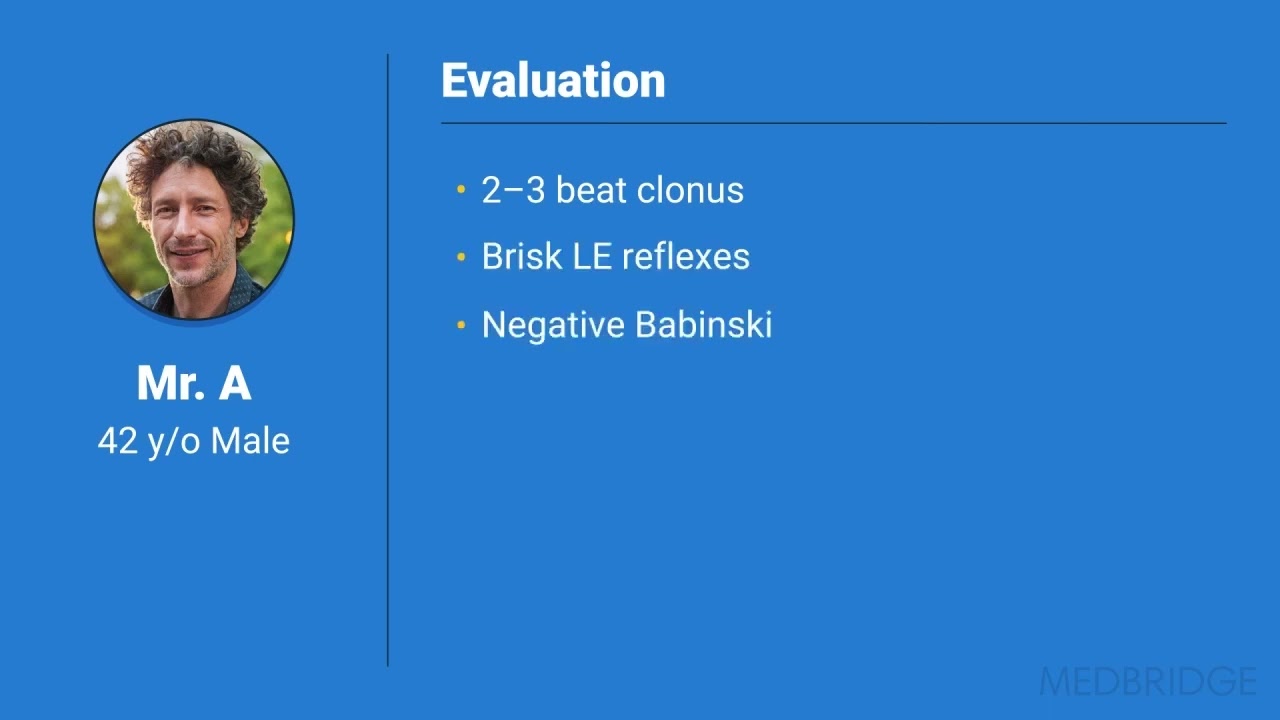
On a case-by-case basis, we've seen some patients with chronic whiplash who also present symptoms of central nervous system involvement. In one case, a 42-year old patient had been involved in a simple rear-end motor vehicle crash. For a little over three years, the patient experienced persistent bodily pain, weakness, depression, and post-traumatic stress.
We found brisk lower extremity reflexes and weakness, but a negative Babinski test, raising the possibility of an injury pointing to a cortico-motor, or upper motor neuron, presentation. We also found profound unilateral changes in lower extremity muscles on MRI, which corresponded to some changes in the imaging of spinal cord pathways on that particular side, so we were seeing some emerging evidence of central nervous system involvement.9,10,11 Not a spinal cord injury per se, but the possibility that something in the spinal cord had been affected, such as the ascending and descending spinal cord white matter pathways.
This is why we think there could be a central or systemic component to whiplash—not in everyone, but in some patients. Performing a few simple clinical tests such as heel raises and reflex evaluation can help you determine if this might be the case for a patient with persistent pain—and if so, you can alter your management strategies accordingly.
3. Identifying Stress-System Dysregulation
Musculoskeletal trauma as part of whiplash-associated disorder (WAD) can be understood as a stressor. Stress can affect people both physiologically and psychologically, and we have found that there is very likely overlap in the mechanisms between these two systems. For example, cortisol—the primary stress hormone of humans and the result of increased activity in the hypothalamic-pituitary-adrenal (HPA) axis—has known effects on systemic inflammation, nociceptor sensitization, and cognitive and sleep patterns, each of which are part of the clinical spectrum referred to as WAD.
While not exclusively focused on WAD, a study we recently performed found potentially important associations between systemic cortisol (such as that found in hair) and clinical presentations such as pain, disability, and distress, in adults with acute post-traumatic MSK injuries.12 These findings suggest that performing early interventions to reduce systemic stress might help prevent the transition to chronic pain and disability.
4. Understanding Molecular Mediators
One last advance to mention is preliminary evidence of molecular mediators predicting persistent neck pain. We've discovered some initial evidence that the microRNA Let-7i-5p (which has been shown to influence adipocyte function in both mice and humans13) mediates the relationship between fatty infiltrates in the neck and persistent neck pain following an MVC.14 We consider this a preliminary breakthrough in our understanding of what's potentially happening at a molecular level in patients with chronic whiplash and an important step toward the ability to more effectively tailor treatment.
A Continuously Evolving Field
As the field of whiplash rehabilitation evolves, exciting opportunities for PTs to refine their approaches continue to present themselves. Understanding the diversity of recovery trajectories, recognizing high-risk factors, and staying informed about the latest advances in prognosis, diagnosis, and treatment are essential components of effective patient care.
References
Ritchie C, Sterling M. Recovery Pathways and Prognosis After Whiplash Injury. J Orthop Sports Phys Ther. 2016 Oct;46(10):851-861. doi: 10.2519/jospt.2016.6918. Epub 2016 Sep 3. PMID: 27594661.
Abbott R, Peolsson A, West J, Elliott JM, slund U, Karlsson A, Leinhard OD. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J. 2018 May;18(5):717-725. doi: 10.1016/j.spinee.2017.08.233. Epub 2017 Sep 5. PMID: 28887274; PMCID: PMC8845185.
Elliott JM, Walton DM, Albin SR, Courtney DM, Siegmund GP, Carroll LJ, Weber KA 2nd, Smith AC. Biopsychosocial sequelae and recovery trajectories from whiplash injury following a motor vehicle collision. Spine J. 2023 Jul;23(7):1028-1036. doi: 10.1016/j.spinee.2023.03.005. Epub 2023 Mar 21. PMID: 36958668; PMCID: PMC10330498.
Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006 Oct 15;31(22):E847-55. doi: 10.1097/01.brs.0000240841.07050.34. PMID: 17047533.
Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, OLeary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine (Phila Pa 1976). 2014 Jan 1;39(1):39-47. doi: 10.1097/BRS.0000000000000033. PMID: 24270932.
Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008 Jun;63(6):681-7. doi: 10.1016/j.crad.2007.11.011. Epub 2008 Jan 31. PMID: 18455560.
Weber KA 2nd, Abbott R, Bojilov V, Smith AC, Wasielewski M, Hastie TJ, Parrish TB, Mackey S, Elliott JM. Multi-muscle deep learning segmentation to automate the quantification of muscle fat infiltration in cervical spine conditions. Sci Rep. 2021 Aug 16;11(1):16567. doi: 10.1038/s41598-021-95972-x. PMID: 34400672; PMCID: PMC8368246.
Wesselink EO, Elliott JM, Coppieters MW, Hancock MJ, Cronin B, Pool-Goudzwaard A, Weber Ii KA. Convolutional neural networks for the automatic segmentation of lumbar paraspinal muscles in people with low back pain. Sci Rep. 2022 Aug 5;12(1):13485. doi: 10.1038/s41598-022-16710-5. PMID: 35931772; PMCID: PMC9355981.
Hoggarth MA, Elliott JM, Smith ZA, Paliwal M, Kwasny MJ, Wasielewski M, Weber KA 2nd, Parrish TB. Macromolecular changes in spinal cord white matter characterize whiplash outcome at 1-year post motor vehicle collision. Sci Rep. 2020 Dec 17;10(1):22221. doi: 10.1038/s41598-020-79190-5. PMID: 33335188; PMCID: PMC7747591.
Smith AC, Parrish TB, Hoggarth MA, McPherson JG, Tysseling VM, Wasielewski M, Kim HE, Hornby TG, Elliott JM. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases. 2015;1:15024. doi: 10.1038/scsandc.2015.24. Epub 2015 Oct 8. Erratum in: Spinal Cord Ser Cases. 2016 Jul 21;2:16019. PMID: 27630770; PMCID: PMC5019487.
Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med. 2014 Nov;15(11):1938-44. doi: 10.1111/pme.12518. Epub 2014 Aug 19. PMID: 25139822; PMCID: PMC6597006.
Jesin JA, Walton DM. Cortisol as a Marker of Pain and Distress After Acute Musculoskeletal Trauma. Clin J Pain. 2024 Mar 1;40(3):157-164. doi: 10.1097/AJP.0000000000001188. PMID: 38168023.
Giroud M, Karbiener M, Pisani DF, Ghandour RA, Beranger GE, Niemi T, Taittonen M, Nuutila P, Virtanen KA, Langin D, Scheideler M, Amri EZ. Let-7i-5p represses brite adipocyte function in mice and humans. Sci Rep. 2016 Jun 27;6:28613. doi: 10.1038/srep28613. PMID: 27345691; PMCID: PMC4921928.
Elliott JM, Rueckeis CA, Pan Y, Parrish TB, Walton DM, Linnstaedt SD. microRNA let-7i-5p mediates the relationship between muscle fat infiltration and neck pain disability following motor vehicle collision: a preliminary study. Sci Rep. 2021 Feb 4;11(1):3140. doi: 10.1038/s41598-021-82734-y. PMID: 33542428; PMCID: PMC7862492.
Below, watch James M. Elliott explain how to identify possible central nervous system involvement in whiplash injuries in this brief clip from his MedBridge course “Whiplash-Associated Disorders: Evidence-Based Practice."